不含 IBOA 的粘合剂
皮肤是人体最大的器官。它可以呼吸、再生,并受其上所放置物品的影响。随着可穿戴设备越来越受欢迎,组装和在某些情况下固定在身体上的材料也需要考虑。
使用诊断和治疗医疗可穿戴设备随着技术的发展,消费者需要通过监测、药物输送和疼痛管理系统以简单的方式管理健康,胰岛素泵正成为主流。生命体征监测设备、睡眠监测器和连续血糖监测器等测量工具可收集用户的运动或个人健康数据,从而轻松跟踪信息,甚至将其发送给医疗保健提供者。胰岛素泵和可穿戴注射器在非临床环境中提供了一种胰岛素和新型药物输送方式。这些设备需要经受反复使用、长时间留在身体上、暴露在不同环境中并能够保持在原位。
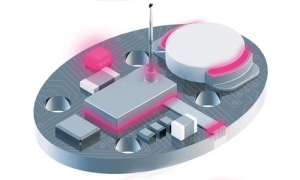
除了用于连接和保护可穿戴设备内部电子元件的粘合剂、涂层和封装剂外,这些材料还用于连接或密封组件内的塑料和金属。此外,粘合剂还可用于将设备固定在皮肤上或与皮肤短暂或长时间接触。
医疗可穿戴设备图表显示了可使用 Dymax 粘合剂组装组件的区域。
有时化学物质 IBOA(丙烯酸异冰片酯)可穿戴设备中含有 IBOA,这是一种已知的皮肤致敏剂。IBOA 是一种单官能活性稀释剂,是制造丙烯酸树脂的关键单体,因为它在暴露于来自能量源(如紫外线)的自由基时会聚合。IBOA 还因其抗冲击性、柔韧性和硬度等特性而用作各种医疗器械塑料中的增塑剂。如果粘合剂或基材的配方中包含 IBOA,则如果未完全固化和交联,则有可能引起皮肤敏感问题。
此外,用于粘合可穿戴设备内部组件的粘合剂、封装剂和涂层可能会在固化过程中释放气体,并重新沉积到可能接触皮肤的其他表面上。如果粘合剂通道外有任何“溢出”,则可能会有一小滴或一部分暴露在外部。应用中未固化的粘合剂也会导致类似的问题。
因此,用户在使用医疗可穿戴设备时可能会出现皮肤敏感的情况。
出于这些原因,参与设计和组装可穿戴设备的制造商必须使用考虑到皮肤敏感性的粘合剂。粘合剂必须不含 IBOA 且通过 ISO 10993评估刺激性和致敏性。其他要考虑的标准是 ISO 10993-5 细胞毒性生物相容性、粘附于低表面能基材的能力以及抗湿气和抗热冲击的能力。
Dymax 生产不含 IBOA 的光固化医疗用胶粘剂符合 ISO 10993-10 标准,并证实完全固化后不会引起皮肤致敏。
要了解有关该系列材料的更多信息或讨论您的应用,请联系我们的应用工程团队。